A Robust, Transformative Pipeline of Wholly Owned Assets1
Adagene’s purpose-built technology platforms have generated a pipeline of potential first or best in class therapeutic assets with unique MOAs.
- ADG116: This NEObody program, targeting a unique epitope of CTLA-4, is being evaluated in patients with advanced/metastatic solid tumors. ADG116 is designed to provide an enhanced efficacy profile by potent Treg depletion in the tumor microenvironment (TME) and to maintain its physiological function by soft ligand blocking to address safety concerns associated with existing CTLA-4 therapeutics. Publications →
- ADG126: This SAFEbody program applies precision masking technology to ADG116 for conditional activation in the TME to expand the therapeutic index and to further address safety concerns with existing CTLA-4 therapies. ADG126 is designed to provide enhanced safety and efficacy profiles due to the combination of the potent Treg depletion in the TME and soft ligand blocking. Interim clinical data in patients with advanced/metastatic solid tumors have shown its compelling safety profile with repeat dosing across dosing levels, as well as an indication of antitumor activity in this heavily pretreated patient population. Publications →
- ADG106: This NEObody program is a fully human ligand-blocking, agonistic anti-CD137 IgG4 monoclonal antibody (mAb) that is being evaluated in patients with advanced solid tumors and/or non-Hodgkin’s lymphoma. Publications →
- Preclinical Programs: Our five differentiated programs in IND-enabling studies are supported by preclinical data showing potential first or best-in-class profiles of these transformative product candidates. Publications →
Global partnerships and investments attest to the strong science underlying our pipeline and its potential to produce novel single-agent and combination therapies.
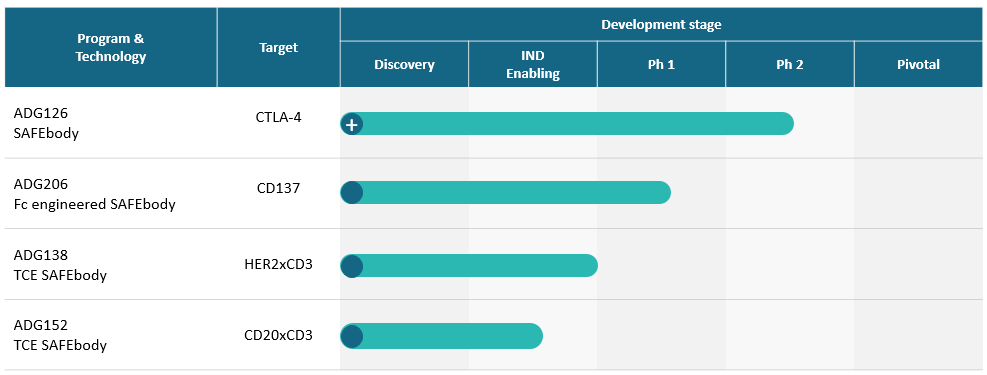
| Program & Technology | Target | Development stage | ||||
|---|---|---|---|---|---|---|
| Discovery | IND Enabling | Ph 1 | Ph 2 | Pivotal | ||
| ADG126 SAFEbody | CTLA-4 | ADG126 is a fully human anti-CTLA-4 SAFEbody designed to address the safety concerns associated with existing CTLA-4 therapeutics. It is designed to enhance the safety features by masking the antibody binding site of ADG126, which would be unmasked in the TME, where the activated ADG126 would block CTLA-4 and deplete regulatory T-cells by means of enhanced antibody-dependent cellular cytotoxicity, or ADCC.
|
||||
| ADG206 Fc engineered SAFEbody | CD137 | Our masked, IgG1 FC-engineered anti-CD137 POWERbody™ combines conditional activation in the tumor microenvironment with strong agonistic activity through heightened FcγR-mediated crosslinking for therapeutic potential in either single agent or combination regimens.
|
||||
| ADG138 TCE SAFEbody | HER2xCD3 | Our HER2xCD3 POWERbody is masked on both arms with an impressively high therapeutic index relative to its parental non-masked TCE in both HER2 high and low expressing solid tumors, supporting its development for HER2-expressing solid tumors as a single agent and in combination with other immune modulating agents.
|
||||
| ADG152 TCE SAFEbody | CD20xCD3 | Our bispecific CD20xCD3 T-cell engager POWERbody integrates SAFEbody precision masking technology to minimize cytokine release syndrome (CRS) and on-target/off-tumor toxicities for an increased therapeutic index.
|
||||
Notes:
1 Two additional candidates derived from Adagene’s AI-powered antibody platform are in development by other entities. These include ADG104, an anti-PD-L1 antibody in phase 2 development by Sanjin, and ADG125, an antibody targeting CSF-1R in phase 1 development by Dragon Boat Biopharmaceutical.

